Controlling inflammation requires a comprehensive clinical approach1
Today’s understanding of inflammation builds on a 50-year history of cytokine research2
Since early cytokine research and the discovery of IL-1, our understanding of inflammation has become more complex. It has expanded to focus on the development of targeted therapies that address the different roles cytokines play in inflammation.
IL=interleukin.
1970s
The 1970s marked the beginning of cytokine research with the discovery of IL-1 in 1974 and TNF in 1975.2
TNF=tumor necrosis factor.
1980s
The 1980s saw rapid advancements in cytokine research, including the identification of IL-6 and the first in vivo studies with biologic treatments targeting TNF.2
1990s
In the 1990s, IL-17 was identified and cloned, revealing a new type of helper T cells, named Th17.3
2000s
In 2000, IL-23 was discovered as a novel member of the IL-12 family, bringing recognition of inflammation’s role in various diseases such as PSO and PsA.4
PsA=psoriatic arthritis; PSO=psoriasis.
2010s
The 2010s saw continued development and approval of therapies targeting IL-17, IL-23, and TNF for treating PSO, PsA, and AS, among others.5
AS=ankylosing spondylitis.

2020s
Recent years have focused on developing targeted therapies and exploring the complex interplay among different cytokines in inflammatory conditions, with an increased understanding of the IL-12/IL-23 pathway, including IL-17A, IL-17F, IL-22, GM-CSF, IL-6, and TNF-α.4,5

Inflammation is the body’s immune response to harmful stimuli6
Inflammation occurs in response to harmful stimuli, such as pathogens, damaged cells, or irritants. Its purpose is to protect the body by eliminating the cause of cell injury, then clearing out damaged cells and tissues to initiate tissue repair.6,7
Triggers of inflammation include pathogens (bacteria, viruses, fungi), physical injuries (cuts, burns, trauma), chemical irritants, foreign bodies (splinters, dirt), or radiation exposure.6
When inflammation occurs, blood vessels dilate to increase blood flow to the affected area. Capillaries become more permeable, allowing fluid and white blood cells to enter tissues. These white blood cells then migrate to the affected area to fight pathogens and remove debris.6,7
The 5 cardinal signs of acute inflammation6
-
Redness
-

Heat
-
Swelling
-

Pain
-
Loss of function
Inflammation can be acute or chronic
An acute inflammatory response is temporary and resolves in days to weeks in a state of health. Inflammation that fails to resolve becomes prolonged or chronic.7
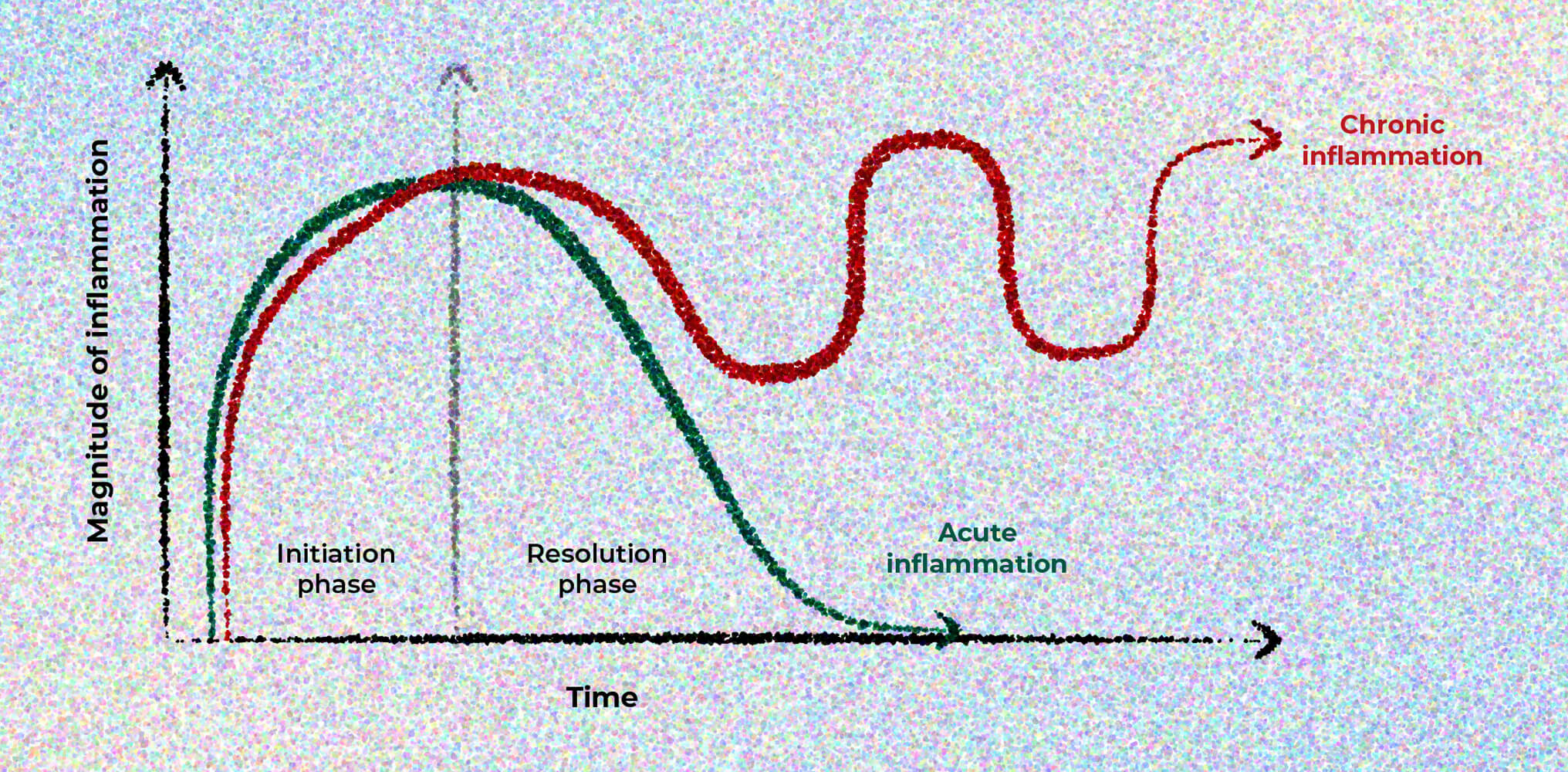
©Oronsky B, Caroen S, Reid T. What exactly is inflammation (and what is it not?). Int J Mol Sci. 2022;23(23):14905. Published 2022 Nov 28. doi:10.3390/ijms232314905. Licensed under CC BY 4.0.
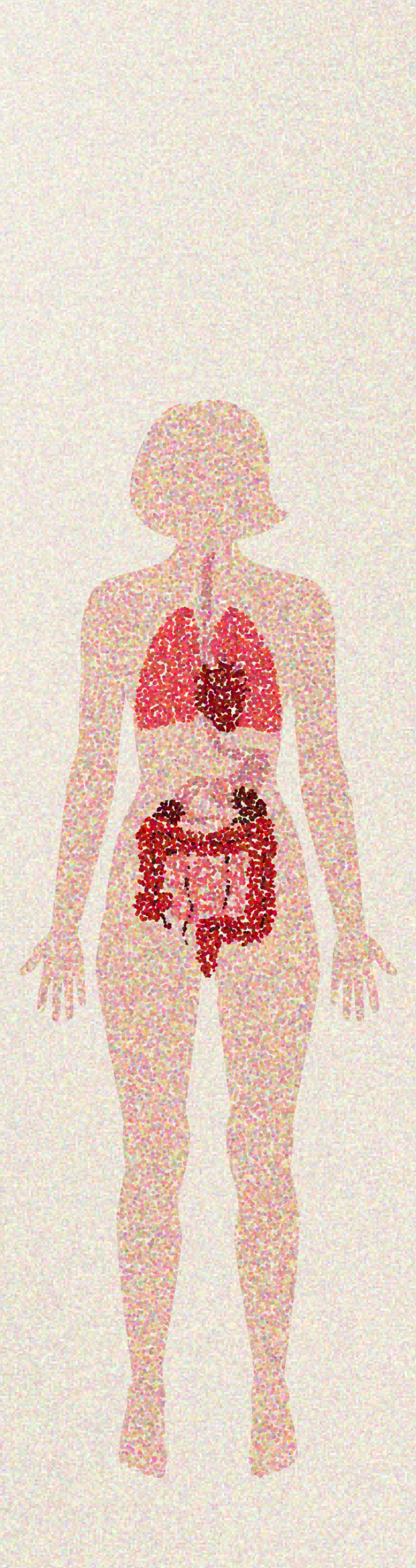
Explore major manifestations of inflammation associated with rheumatic diseases
Cardiovascular
Cardiovascular disease is the most common cause of death in patients with RA. Inflammation in RA predisposes patients to developing cardiovascular disease and contributes to the risk of mortality and disability. RA shares genetic and environmental factors with atherosclerosis that may cause endothelial dysfunction.8
RA=rheumatoid arthritis.
Pulmonary
Pulmonary manifestations include asthma, COPD, and ILD—making respiratory disease the second major cause of death in patients with RA.8
COPD=chronic obstructive pulmonary disease; ILD=interstitial lung disease.
Ocular
Ocular manifestations (especially in SpA) include uveitis, scleritis, and episcleritis.9
SpA=spondyloarthritis.
Cutaneous
Cutaneous manifestations include rheumatoid nodules (in RA), psoriasis (in PsA), rashes (in lupus), and vasculitis (in RA).5,8,10
Gastrointestinal
Gastrointestinal manifestations include IBD (ulcerative colitis and Crohn’s disease). IBD has been associated with spondyloarthropathies.11
IBD=inflammatory bowel disease.
Uncover the effects of inflammation on quality of life
-
Chronic pain
Chronic or late-stage inflammation can cause chronic pain.12
- Chronic pain is a common manifestation of inflammation in a number of immunologic diseases including RA, axSpA, PSO, PsA, and IBD13-16
- For many of these diseases, pain is typically worse at night and may disrupt sleep13,14,17
axSpA=axial spondyloarthritis.
-

Fatigue
Inflammation and its consequences can cause fatigue.18-20
- 70% of patients with RA experience severe fatigue19
- Patients with axSpA and PsA may still experience fatigue, even after achieving low disease activity status21
-
Mobility
Inflammation can influence patients’ mobility and physical function.
- Inflammation can lead to reduced spinal mobility over time in axSpA21,22
- In patients with AS, reduced physical function is associated with lower quality of life23,24
Impact on quality of life
Pain severity, disability, and psychological factors all contribute to decreased quality of life.13 Patients with axSpA report reduced health-related quality of life and work productivity compared to the general population.25
The inflammatory disease process in axSpA and PsA affects multiple aspects of patients’ lives. These effects may include an impaired ability to work and increased risk of depression.25-28 Extra-articular manifestations like uveitis and IBD further reduce quality of life.15,29,30
The mechanisms linking inflammation to these symptoms are complex. Inflammatory cytokines can directly affect the brain, altering neurotransmitter systems and neural circuits involved in fatigue, mood, and pain processing.12,19
The role of pro-inflammatory cytokines
Pro-inflammatory cytokines amplify the inflammatory cascade and recruit immune cells to the sites of inflammation.31,32
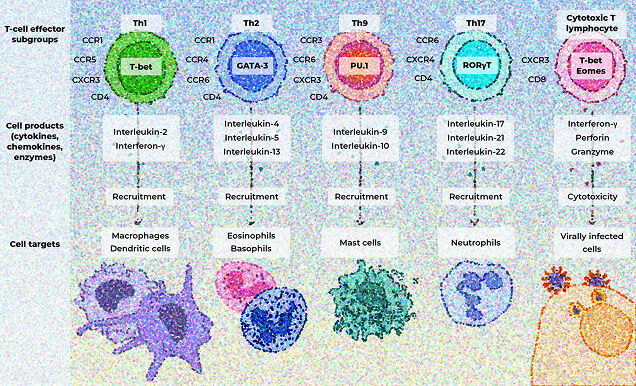
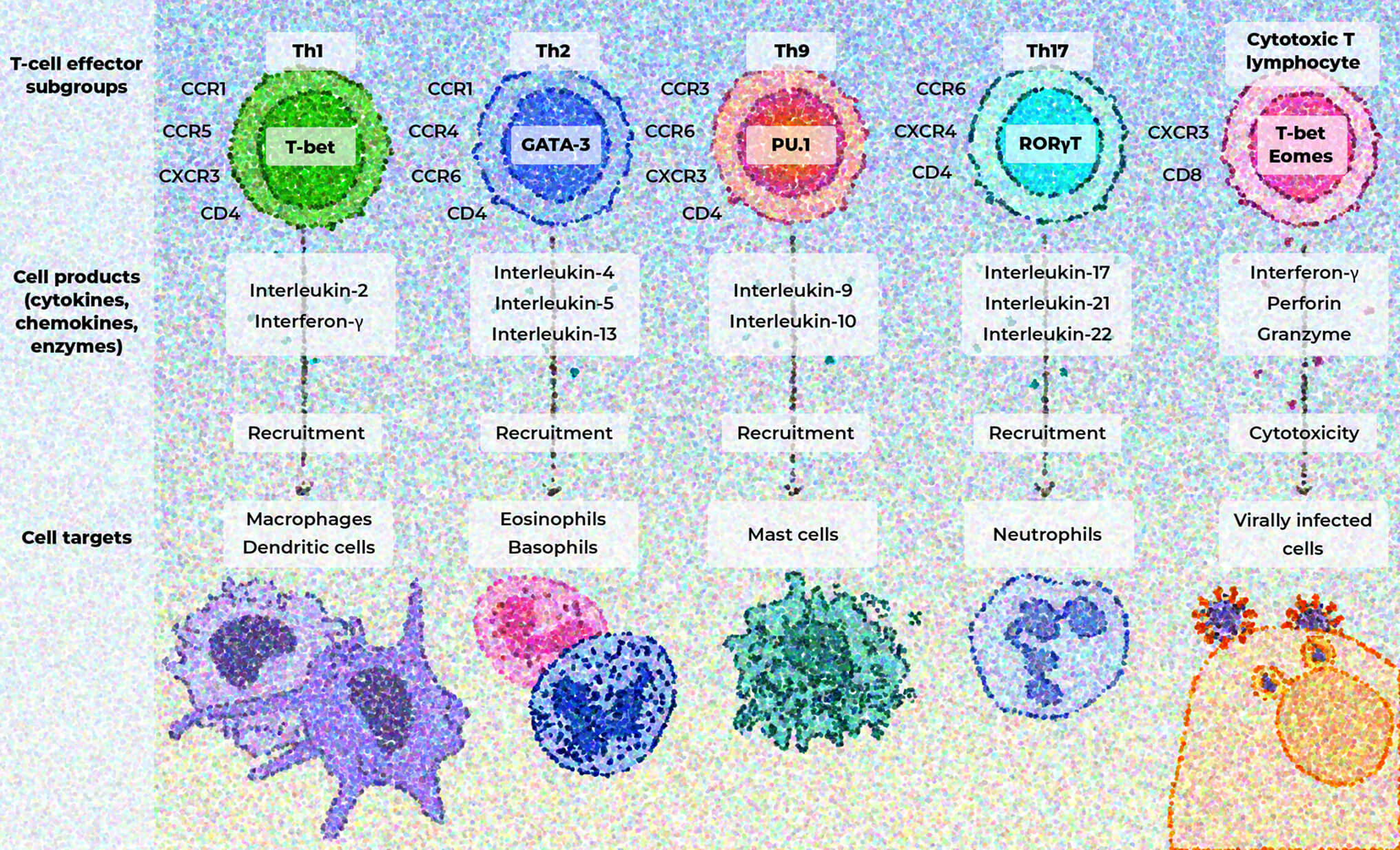
T-cell subgroups release cytokines, chemokines, and enzymes that activate pro-inflammatory signaling pathways by recruiting immune cells that drive further inflammation and contribute to tissue damage and organ dysfunction.33
Spotlight on the amplifying role of IL-17
IL-17 often acts synergistically with other inflammatory mediators, amplifying inflammatory responses.31,34-36
IL-17 has diverse effects in different tissues34,36,37:

Joints
IL-17 is involved in bone degradation and chronic joint inflammation.36 IL-17A and IL-17F have been shown to act synergistically to promote bone remodeling and tissue inflammation, highlighting the role of IL-17 in disease pathogenesis.10,36
Dysregulated IL-17 responses contribute to various autoimmune and inflammatory disorders, which may include PSO, PsA, RA, axSpA, and IBD.36,38
Explore the immunological cascade and the role of IL-17 in immune-mediated disease


Skin
IL-17 promotes keratinocyte proliferation and inflammatory mediator production in conjunction with IL-22, and has been shown to play a role in the pathogenesis of psoriatic disease.10,36
Dysregulated IL-17 responses contribute to various autoimmune and inflammatory disorders, which may include PSO, PsA, RA, axSpA, and IBD.36,38
Explore the immunological cascade and the role of IL-17 in immune-mediated disease
Dive deeper into the roles of IL-17A and IL-17F in the pathogenesis of inflammation
The interplay of immune mediators in inflammation is complex and involves a variety of cytokines, chemokines, and other inflammatory mediators.10


TAKE YOUR SEATS IN THE PATHOBIOLOGY LEARNING THEATRE
Uncover how IL-17A and IL-17F work in tandem in the inflammation of SpA
Become a member of the RheuMuseum and be the first to know about new exhibits
Sign up to stay in the loop on new exhibits about clinical research, important updates, and other educational opportunities.
- Pahwa R, Goyal A, Jialal I. Chronic inflammation [Updated August 7, 2023]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493173/
- Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008. doi:10.3390/ijms20236008
- Wu X, Tian J, Wang S. Insight into non-pathogenic Th17 cells in autoimmune diseases. Front Immunol. 2018;9:1112. doi:10.3389/fimmu.2018.01112
- Krueger JG, Eyerich K, Kuchroo VK, et al. IL-23 past, present, and future: a roadmap to advancing IL-23 science and therapy. Front Immunol. 2024;15:1331217. doi:10.3389/fimmu.2024.1331217
- Song Y, Li J, Wu Y. Evolving understanding of autoimmune mechanisms and new therapeutic strategies of autoimmune disorders. Signal Transduct Target Ther. 2024;9(1):263. doi:10.1038/s41392-024-01952-8
- In brief: what is an inflammation? InformedHealth.org. May 18, 2021. Accessed February 14, 2025. https://www.ncbi.nlm.nih.gov/books/NBK279298/
- Oronsky B, Caroen S, Reid T. What exactly is inflammation (and what is it not?). Int J Mol Sci. 2022;23(23):14905. doi:10.3390/ijms232314905
- Figus FA, Piga M, Azzolin I, et al. Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmun Rev. 2021;20(4):102776. doi:10.1016/j.autrev.2021.102776
- Rademacher J, Poddubnyy D, Pleyer U. Uveitis in spondyloarthritis. Ther Adv Musculoskelet Dis. 2020;12:1759720X20951733. doi:10.1177/1759720X20951733
- Zalesak M, Danisovic L, Harsanyi S. Psoriasis and psoriatic arthritis-associated genes, cytokines, and human leukocyte antigens. Medicina (Kaunas). 2024;60(5):815. doi:10.3390/medicina60050815
- Zioga N, Kogias D, Lampropoulou V, et al. Inflammatory bowel disease-related spondyloarthritis: the last unexplored territory of rheumatology. Mediterr J Rheumatol. 2022;33(suppl 1):126-136. doi:10.31138/mjr.33.1.126
- Fang XX, Zhai MN, Zhu M, et al. Inflammation in pathogenesis of chronic pain: foe and friend. Mol Pain. 2023;19:17448069231178176. doi:10.1177/17448069231178176
- Magrey M, Walsh JA, Flierl S, et al. The International Map of Axial Spondyloarthritis survey: a US patient perspective on diagnosis and burden of disease. ACR Open Rheumatol. 2023;5(5):264-276. doi:10.1002/acr2.11543
- Walsh DA, McWilliams DF. Pain in rheumatoid arthritis. Curr Pain Headache Rep. 2012;16(6):509-517. doi:10.1007/s11916-012-0303-x
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37. doi:10.1016/j.jaut.2016.10.009
- Bakshi N, Hart AL, Lee MC, et al. Chronic pain in patients with inflammatory bowel disease. Pain. 2021;162(10):2466-2471. doi:10.1097/j.pain.0000000000002304
- Pathan EMI, Inman RD. Pain in spondyloarthritis: a neuro-immune interaction. Best Pract Res Clin Rheumatol. 2017;31(6):830-845. doi:10.1016/j.berh.2018.07.003
- Lacourt TE, Vichaya EG, Chiu GS, et al. The high costs of low-grade inflammation: persistent fatigue as a consequence of reduced cellular-energy availability and non-adaptive energy expenditure. Front Behav Neurosci. 2018;12:78. doi:10.3389/fnbeh.2018.00078
- Morris G, Berk M, Walder K, et al. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. doi:10.1186/s12916-014-0259-2
- Skougaard M, Jørgensen TS, Rifbjerg-Madsen S, et al. Relationship between fatigue and inflammation, disease duration, and chronic pain in psoriatic arthritis: an observational DANBIO Registry study. J Rheumatol. 2020;47(4):548-552. doi:10.3899/jrheum.181412
- Liu V, Fong W, Kwan YH, et al. Residual disease burden in patients with axial spondyloarthritis and psoriatic arthritis despite low disease activity states in a multiethnic Asian population. J Rheumatol. 2021;48(5):677-684. doi:10.3899/jrheum.200934
- Calvo-Gutierrez J, Garrido-Castro JL, Gil-Cabezas J, et al. Is spinal mobility in patients with spondylitis determined by age, structural damage, and inflammation? Arthritis Care Res (Hoboken). 2015;67(1):74-79. doi:10.1002/acr.22400
- Torre-Alonso JC, Queiro R, Comellas M, et al. Patient-reported outcomes in European spondyloarthritis patients: a systematic review of the literature. Patient Prefer Adherence. 2018;12:733-747. doi:10.2147/PPA.S162420
- Vesović-Potić V, Mustur D, Stanisavljević D, et al. Relationship between spinal mobility measures and quality of life in patients with ankylosing spondylitis. Rheumatol Int. 2009;29(8):879-884. doi:10.1007/s00296-008-0759-5
- da Silva AB, Ramiro S, Boel A, et al. Do quality of life and work productivity change in early axial spondyloarthritis and non-axial spondyloarthritis patients after two years? Rheumatology (Oxford). doi:10.1093/rheumatology/keae346
- Park JY, Howren AM, Zusman EZ, et al. The incidence of depression and anxiety in patients with ankylosing spondylitis: a systematic review and meta-analysis. BMC Rheumatol. 2020;4:12. doi:10.1186/s41927-019-0111-6
- Gossec L, Humphries B, Rutherford M, et al. Improvement in work productivity among psoriatic arthritis patients treated with biologic or targeted synthetic drugs: a systematic literature review and meta‑analysis. Arthritis Res Ther. 2024;26(1):50. doi:10.1186/s13075-024-03282-0
- Coates LC, Soriano ER, Corp N, et al.; and the GRAPPA Treatment Recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465-479. doi:10.1038/s41584-022-00798-0
- O'Rourke M, Haroon M, Alfarasy S, et al. The effect of anterior uveitis and previously undiagnosed spondyloarthritis: results from the DUET cohort. J Rheumatol. 2017;44(9):1347-1354. doi:10.3899/jrheum.170115
- van der Meer R, Arends S, Kruidhof S, et al. Extraskeletal manifestations in axial spondyloarthritis are associated with worse clinical outcomes despite the use of tumor necrosis factor inhibitor therapy. J Rheumatol. 2022;49(2):157-164. doi:10.3899/jrheum.210308
- Kuwabara T, Ishikawa F, Kondo M, et al. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017;2017:3908061. doi:10.1155/2017/3908061
- Ge Y, Huang M, Yao YM. Biology of interleukin-17 and its pathophysiological significance in sepsis. Front Immunol. 2020;11:1558. doi:10.3389/fimmu.2020.01558
- Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255-2273. doi:10.1056/NEJMra2026131
- Zhao J, Chen X, Herjan T, et al. The role of interleukin-17 in tumor development and progression. J Exp Med. 2020;217(1):e20190297. doi:10.1084/jem.20190297
- Liu X, Sun S, Liu D. IL-17D: a less studied cytokine of IL-17 family. Int Arch Allergy Immunol. 2020;181(8):618-623. doi:10.1159/000508255
- Brembilla NC, Senra L, Boehncke WH. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front Immunol. 2018;9:1682. doi:10.3389/fimmu.2018.01682
- Miossec P. Update on interleukin-17: a role in the pathogenesis of inflammatory arthritis and implication for clinical practice. RMD Open. 2017;3:e000284. doi:10.1136/rmdopen-2016-000284
- Wang R, Maksymowych WP. Targeting the interleukin-23/interleukin-17 inflammatory pathway: successes and failures in the treatment of axial spondyloarthritis. Front Immunol. 2021;12:715510. doi:10.3389/fimmu.2021.71551