
Explore how immune dysregulation drives inflammation in patients with axSpA
The role of different immune cells in axSpA
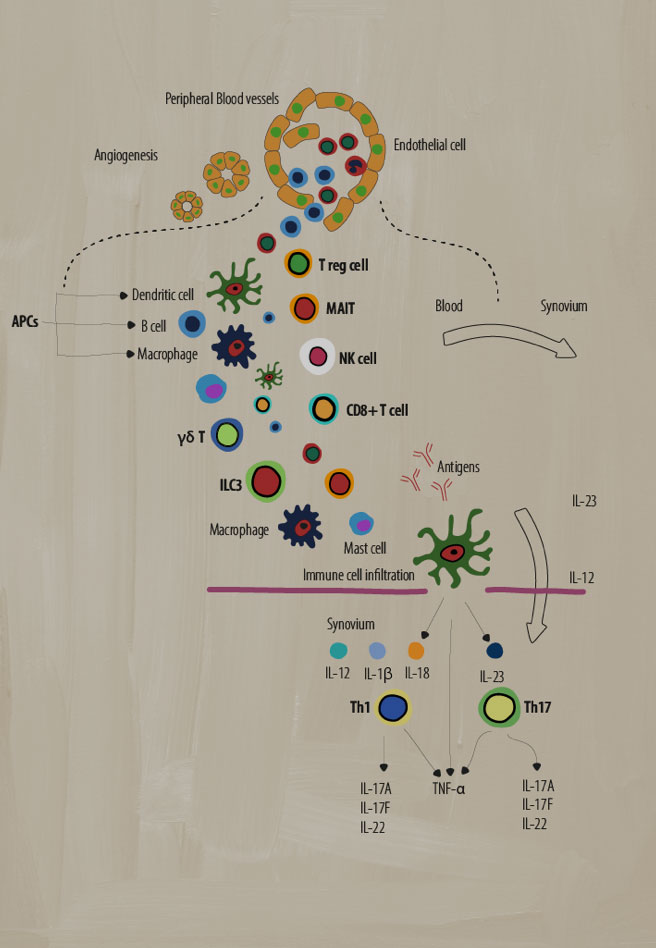
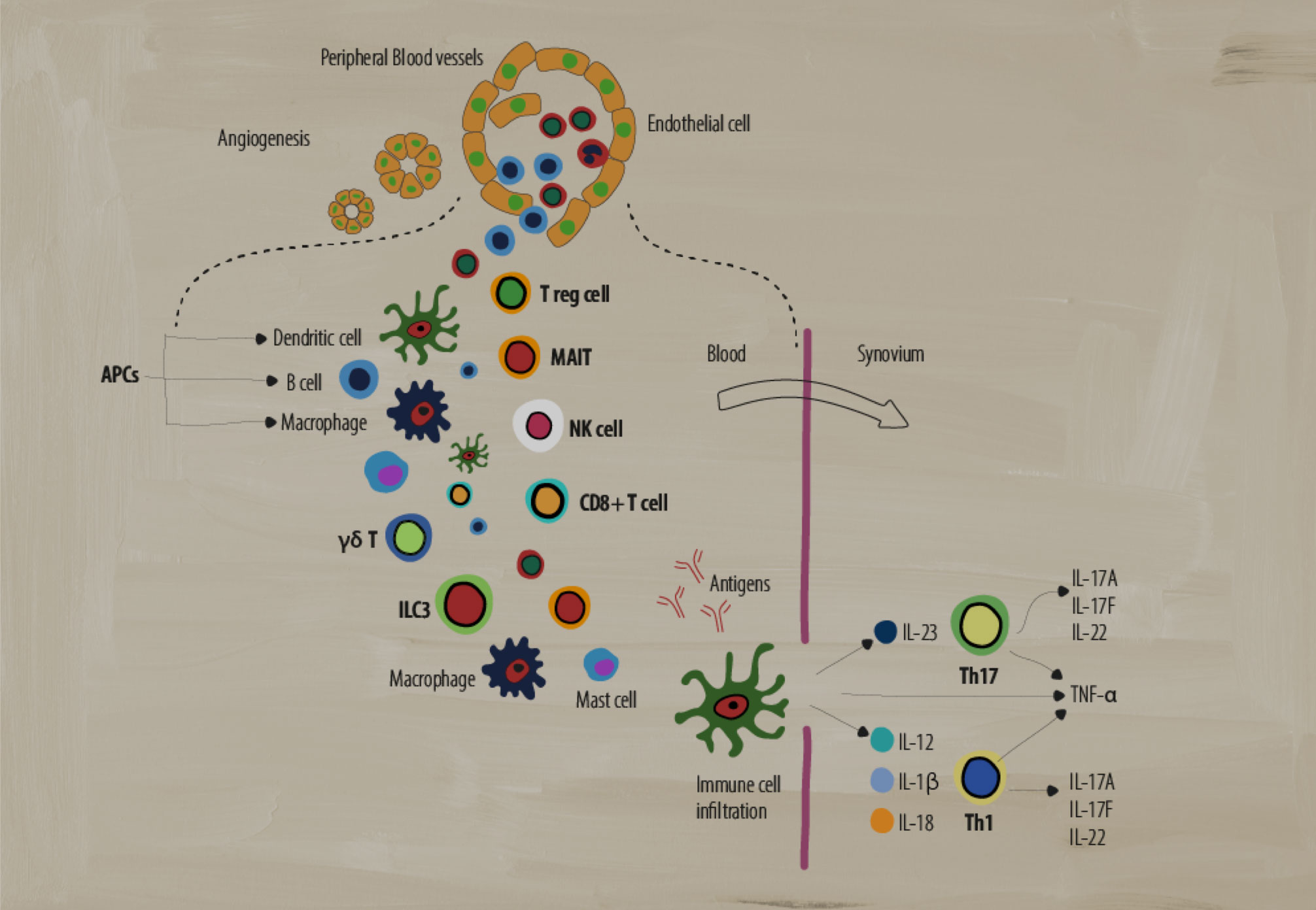
A dysregulated immune system has many moving parts that act to create the chronic inflammation indicative of disease pathology in SpA. Several types of adaptive and innate immune cells play key roles in mediating inflammation.1-4
Download a key to help guide you through each piece
- Innate
- Adaptive
Antigen presenting cells (APCs)
What are they? APCs include B cells, macrophages, and dendritic cells (DCs) that are involved in cellular immunity. They process and present antigens for recognition by other elements of the immune system, such as T cells.5
Role in SpA:
Dendritic cells activate the adaptive immune response by stimulating Th1 and Th2 inflammatory responses while monocyte-derived DCs have been implicated in dysregulated signaling pathways that cause inflammatory responses in Th17 cells.2 IL-23 and IL-12 cytokines secreted by DCs are thought to drive the differentiation of Th17 and Th1 subsets of T cells.4
Macrophages can produce high levels of many pro-inflammatory cytokines, such as IL-12, IL-6, IL-23, and TNF-α, that perpetuate chronic inflammation.4 For example, activated macrophages produce IL-23, which promotes TH17 cell differentiation.4 These cells are found in higher numbers in the synovium and entheses, correlating with SpA disease activity.2
Gamma-delta T cells (γẟ T)
What are they? A subgroup of T cells with the distinct T-cell receptor (TCR) γ and δ chains on their cell surface that act in an innate-like fashion. They are known for non-MHC restricted antigen recognition and abundant cytokine secretion, with some subsets producing IL-17 in the absence of TCR activation.7
Role in SpA: In SpA, there is an increase in IL-23+ γδ T cells heavily biased toward IL-17 production in response to IL-23 or anti-CD3/CD28 signaling.7 Also, increases in IL-17 and GM-CSF double-producing γδ T cells have been found in the peripheral blood of SpA patients.7
MHC=major histocompatibility complex.
Type 3 innate lymphoid cells (ILC3)
What are they? Effector cells in the innate immune system that lack rearranged antigen receptors normally expressed by adaptive cells. Primarily residing in peripheral tissue, they respond to environmental stress and are involved in tissue remodeling, pathogen defense, and maintenance of homeostasis.6
Role in SpA: ILC3 cells are similar to Th17 cells and produce IL-17, IL-22, and GM-CSF. In SpA, ILC3 cell numbers increase and respond to IL-23 and IL-1b by producing IL-17 cytokines, further potentiating a pro-inflammatory state at various sites of clinical manifestation (e.g., entheses).6
T regulatory cells
What are they? A subpopulation of T cells that can regulate and suppress the immune response and play a crucial role in maintaining self-tolerance by the inhibition of T cell activation/proliferation as well as cytokine production.9
Role in SpA: The role of Treg cells in SpA is still under debate. Some studies have shown lower percentages of Tregs and impaired Treg function in the peripheral blood of patients with AS that could represent an imbalance between Tregs and the normal adaptive immune response.10
Treg=T regulatory cells.
Mucosal-associated invariant T cell (MAIT)
What are they? Unique “innate-like” T cells that have functionality across both innate and adaptive immunity.8 These cells exhibit an effector-memory phenotype and produce a wide range of cytokines/molecules that induce cytotoxic and pro-inflammatory responses.8
Role in SpA: In SpA, MAIT cells can induce IL-17, IFN-γ, and granzyme B production in the synovium as well as increase IL-17 and IL-22 production by MAIT cells in the circulation, potentiating a pro-inflammatory state.4,8
Natural killer cells (NK)
What are they? A vital part of the innate immune system, using enzymatic granules to destroy bacteria, viruses, and cancer cells without prior activation. NK cells can be divided into two major groups (dim/bright) based on CD56 expression.2
Role in SpA: In SpA, there is a significant upregulation in the number of CD56dim NK cells. There is a shift in the balance of cell-surface receptors on NK cells that recognize HLA-B27.2 Normally, KIR3DL1 receptors are more prevalent on NK cells, leading to the inhibition of NK cytolytic functionality. In SpA, there were fewer KIR3DL1 receptors than healthy controls.2 NK cells can also secrete pro-inflammatory cytokines such as TNF-α and IFN-γ,which have been shown to play a role in SpA.4
CD8+ T cells
What are they? CD8+ T cells are an important part of the adaptive immune system. They have a variety of functions, such as the production of pro-inflammatory cytokines, the production of cytotoxic granules (like NK cells), and Fas/Fas-ligand signaling.2
Role in SpA: In SpA, CD8+ T cells produce pro-inflammatory cytokines such as TNF-α, IFN-γ, and IL-17, which potentiates a chronic inflammatory state.2 CD8+ T cells also lyse cells through perforin/granzyme (cytotoxic granule) secretion and Fas/Fas-ligand signaling.2
T-helper 17 cells (Th17)
What are they? A subset of CD4+ T cells characterized by their production of the pro-inflammatory cytokine IL-17 that can produce other pro-inflammatory cytokines such as IL-6, IL-26, and IFN-γ.2
Role in SpA: In SpA, elevated levels of IL-23 can induce Th17 cell production.2 High numbers of Th17 cells can cause an overproduction of IL-17, further potentiating a pro-inflammatory state, including production of other inflammatory cytokines such as IL-22 and TNF-α.2,4 Elevated levels of IL-17 can also induce the production of other pro-inflammatory mediators from a variety of cell types such as fibroblasts, endothelial cells, dendritic cells, and macrophages.2
T-helper cells (Th1)
What are they? A subset of CD4+ T cells that play an important role in the identification and destruction of viral and bacterial pathogens.2 These cells secrete pro-inflammatory cytokines such as interferon gamma (IFN-γ), IL-2, and TNF-α to activate other targeted immune cells and initiate immune responses.2
Role in SpA: The role of these cells in SpA is still debated. Some studies show no differences in Th1 cell numbers and pro-inflammatory IFN-γ production, while other studies showed increases in IFN-γ producing CD4+ T cells and increased levels of IFN-γ and TNF-α in patient sera.2
Cytokine dysregulation in axSpA
Following an injury in the entheses, local cells produce chemo-attractants as mediators of inflammation. This causes local immune cells and innate lymphocytes to secrete cytokines. They enter the circulation and recruit T cells and innate immune cells into the inflamed tissue and produce pro-inflammatory and regulatory cytokines in the entheseal site. In a balanced state, this results in regulated and effective repair mechanisms. However, in axSpA, this process is not balanced, and cytokine dysregulation occurs.1,11-13
Cytokine dysregulation is characterized by an excess of inflammatory and/or a depletion of regulatory cytokines, which can lead to pathogenic bone formation. The cytokine balance is influenced by genetic factors, the microbiome, as well as environmental factors, many of which are still unknown.11,13
Genetic risk factors
One of the key genetic risk factors in axSpA is the strong association of AS with inheritance of the HLA allele B27 (HLA-B27).14 HLA-B27 may contribute to inflammatory cytokine production in axSpA through the unfolded protein response.14
Influence of the microbiome
In axSpA, there is an association between the microbiome and disease development.15 A large proportion of IL-17 producing and T regulatory cells are derived from the gut and a different microbiome signature has been observed in patients with AS compared with healthy individuals.15
Sites of inflammation
Different cytokines have been linked with different sites and have distinct roles in different tissues.16
-

Joint
TNF, IL-17, IL-23p19, IL-12/23p40
-

Axial skeleton
IL-17, TNF
-
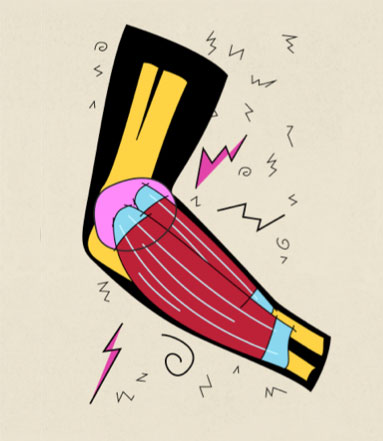
Enthesis
IL-17, IL-12/23p40, TNF, IL-23p19
-
Skin
IL-17, IL-23p19, IL-12/23p40, TNF
-
Gut
IL-12/23p40, TNF, IL-23p19
© Reproduced from Siebert S, Millar NL, McInnes IB. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis. 2019;78(8):1015-1018 with permission from BMJ Publishing Group Ltd.
Learn more about key cytokines that could be dysregulated, leading to inflammation


NEXT ROOM IN THE PATHOBIOLOGY EXHIBIT
Bone damage
Explore the complex pathogenic processes that can lead to bone damage in patients with axSpA.
PREVIOUS ROOM
The Pathobiology Learning Theatre
Become a member of the RheuMuseum and be the first to know about new exhibits
- Furst DE, Louie JS. Targeting inflammatory pathways in axial spondyloarthritis. Arthritis Res Ther. 2019;21(1):135. Published 2019. doi:10.1186/s13075-019-1885-z
- Rezaiemanesh A, Abdolmaleki M, Abdolmohammadi K, et al. Immune cells involved in the pathogenesis of ankylosing spondylitis. Biomed Pharmacother. 2018;100:198-204. doi:10.1016/j.biopha.2018.01.108
- Rosine N, Miceli-Richard C. Innate cells: the alternative source of IL-17 in axial and peripheral spondyloarthritis?. Front Immunol. 2021;11:553742. Published 2021. doi:10.3389/fimmu.2020.553742
- Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273-2284. doi:10.1016/S0140-6736(18)30830-4
- Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14(11):719-730. doi:10.1038/nri3754
- Fang W, Zhang Y, Chen Z. Innate lymphoid cells in inflammatory arthritis. Arthritis Res Ther. 2020;22(1):25. doi: 10.1186/s13075-020-2115-4 181
- Bank I. The role of gamma delta T cells in autoimmune rheumatic diseases. Cells. 2020;9(2):462. Published 2020. doi:10.3390/cells9020462
- Toubal A, Nel I, Lotersztajn S, Lehuen A. Mucosal-associated invariant T cells and disease. Nat Rev Immunol. 2019;19(10):643-657. doi:10.1038/s41577-019-0191-y
- Shevyrev D, Tereshchenko V. Treg Heterogeneity, Function, and Homeostasis. Front Immunol. 2020;10:3100. Published 2020. doi:10.3389/fimmu.2019.03100
- Lai NL, Zhang SX, Wang J, et al. The proportion of regulatory T cells in patients with ankylosing spondylitis: A Meta-Analysis. J Immunol Res. 2019;2019:1058738. Published 2019. doi:10.1155/2019/1058738
- Watad A, Bridgewood C, Russell T, et al. The early phases of ankylosing spondylitis: emerging insights from clinical and basic science. Front Immunol. 2018;9:2668. doi: 10.3389/fimmu.2018.02668
- McGonagle DG, McInnes IB, Kirkham BW, et al. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann Rheum Dis. 2019;78:1167–1178
- Watad A, Rowe H, Russell T, et al. Normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression. Ann Rheum Dis. 2020;79(8):1044-1054. doi:10.1136/annrheumdis-2020-217309
- Simone D, Al Mossawi MH, Bowness P. Progress in our understanding of the pathogenesis of ankylosing spondylitis. Rheumatology (Oxford). 2018;57(suppl_6):vi4-vi9. doi:10.1093/rheumatology/key001
- Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360:j5145. doi:10.1136/bmj.j5145
- Siebert S, Millar NL, McInnes IB. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis. 2019;78(8):1015-1018. doi:10.1136/annrheumdis-2018-213654