

TOUR THE FINAL AXSPA EXHIBIT
axSpA disease outcome measures
Explore different disease outcome measures…
…and their importance in defining axSpA disease activity in clinical practice.
axSpA=axial spondyloarthritis.


LECTURE SERIES WITH DR. ALEXIS OGDIE
Gain insights about suboptimal disease outcomes and raising the standard of care for axSpA.
Watch the first video in a lecture series featuring Dr. Alexis Ogdie—distinguished professor, researcher, and clinician in rheumatology—and discover how consistently using clinical assessment measures, combined with understanding a patient’s goals, can lead to better axSpA disease outcomes.
Many patients with axSpA are not reaching optimal disease outcome goals in clinical practice1-3
~35%-80% of patients do not achieve ASDAS low disease activity1,3*
*ASDAS low disease defined as ASDAS <2.1
ASDAS=Ankylosing Spondylitis Disease Activity Score.
~75%-90% of patients do not achieve ASDAS inactive disease1,2*
*ASDAS inactive disease defined as ASDAS <1.3
Barriers to achieving optimal disease outcomes in axSpA
The decision-making pathway for clinicians is complex. There are multiple reasons why patients do not achieve optimal disease activity goals.4
Challenges in axSpA disease management include:
- Heterogeneous disease manifestations5-7
- Delayed diagnosis, no standard diagnostic tests, and prolonged symptom duration8-10
- No consensus on preferred disease activity measure11
- Patient and clinician discordance on most important disease outcomes12
A recent study showed that only ~20% of patients with axSpA were routinely assessed using composite outcome measures in clinical practice11
There is an opportunity to address this unmet need in the management of patients with axSpA.11
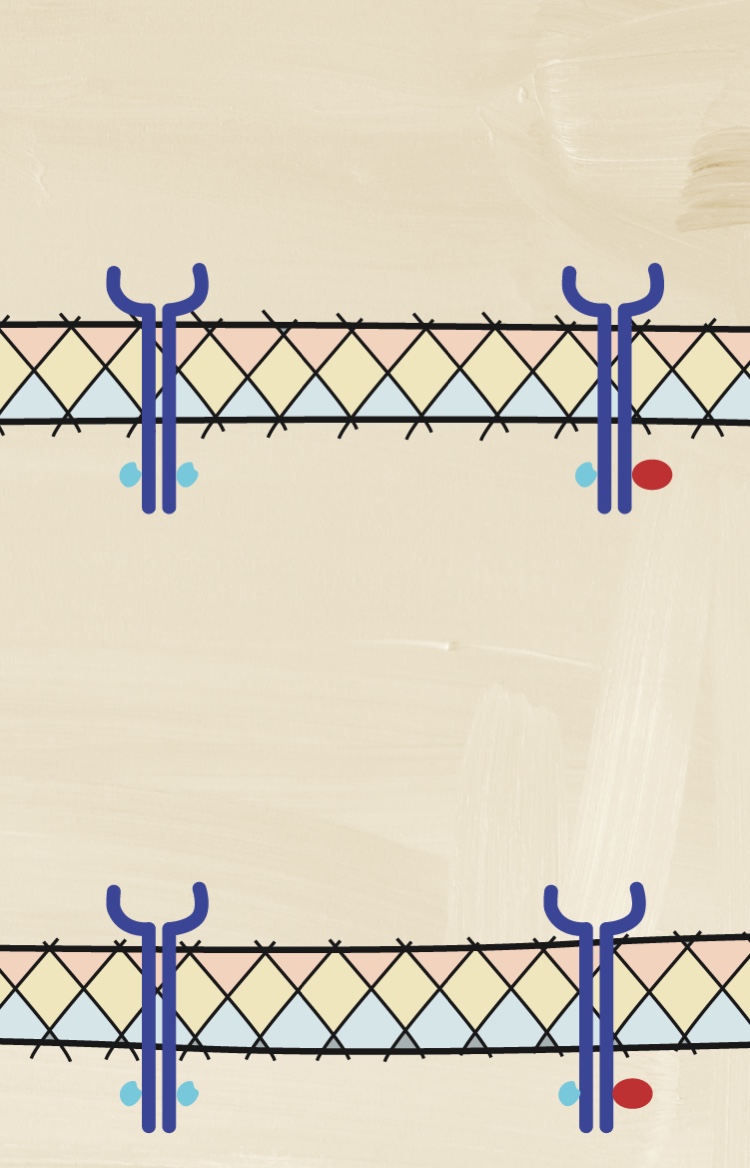
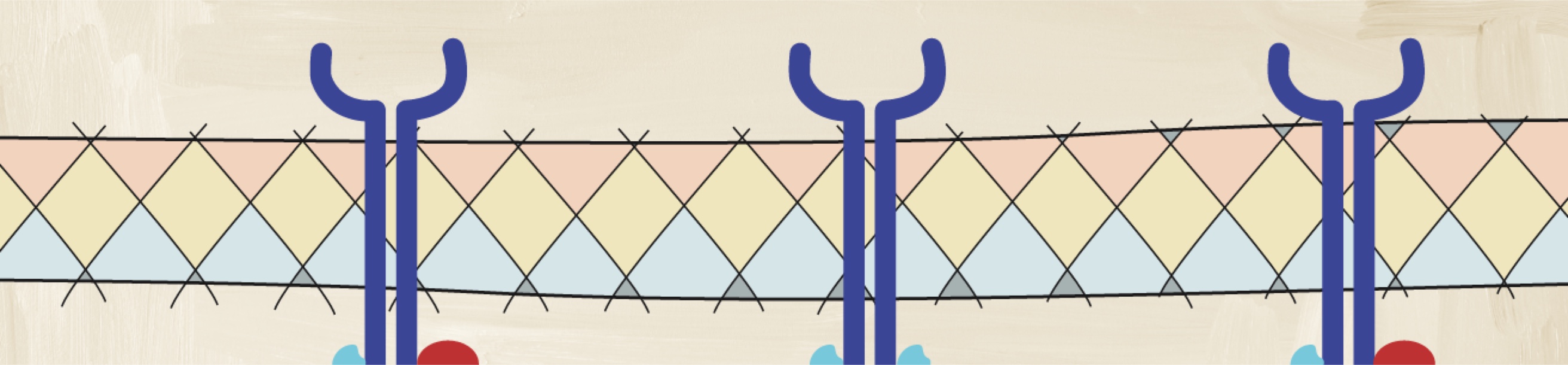
How does the complexity of pathobiology affect the decision-making pathway?
Understanding the pathologic processes leading to axSpA is important to assessing and achieving improved disease outcomes.
NEXT ROOM IN THE OUTCOMES EXHIBIT
Setting higher goals
Explore how the implementation of more stringent measures of axSpA disease activity is key to optimizing disease outcomes.
PREVIOUS EXHIBIT
axSpA manifestations
Become a member of the RheuMuseum and be the first to know about new exhibits
- Michelsen B, Lindström U, Codreanu C, et al. Drug retention, inactive disease and response rates in 1860 patients with axial spondyloarthritis initiating secukinumab treatment: routine care data from 13 registries in the EuroSpA collaboration. RMD Open. 2020;6(3) doi:10.1136/rmdopen-2020-001280
- Ørnbjerg LM, Brahe CH, Askling J, et al. Treatment response and drug retention rates in 24 195 biologic-naïve patients with axial spondyloarthritis initiating TNFi treatment: routine care data from 12 registries in the EuroSpA collaboration. Ann Rheum Dis. 2019;78(11):1536-1544. doi:10.1136/annrheumdis-2019-215427
- Michelena X, Zhao SS, Dubash S, et al. Similar biologic drug response regardless of radiographic status in axial spondyloarthritis: data from the British Society for Rheumatology Biologics Register in Ankylosing Spondylitis registry. Rheumatology. 2021;60(12):5795-5800. doi:10.1093/rheumatology/keab070
- Garg N, van den Bosch F, Deodhar A. The concept of spondyloarthritis: where are we now? Best Pract Res Clin Rheumatol. 2014;28(5):663-72. doi:10.1016/j.berh.2014.10.007
- Navarro-Compán V, Sepriano A, El-Zorkany B, et al. Axial spondyloarthritis. Ann Rheum Dis. 2021;80(12):1511-1521. doi:10.1136/annrheumdis-2021-221035
- Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374(26):2563-74. doi:10.1056/NEJMra1406182
- López-Medina C, Dougados M, Ruyssen-Witrand A, et al. Evaluation of concomitant peripheral arthritis in patients with recent onset axial spondyloarthritis: 5-year results from the DESIR cohort. Arthritis Res Ther. 2019;21(1):139. doi:10.1186/s13075-019-1927-6
- Zhao SS, Pittam B, Harrison NL, et al. Diagnostic delay in axial spondyloarthritis: a systematic review and meta-analysis. Rheumatology. 2021;60(4):1620-1628. doi:10.1093/rheumatology/keaa807
- Poddubnyy D, Sieper J. Current unmet needs in spondyloarthritis. Curr Rheumatol Rep. 2019;21(9):43. doi:10.1007/s11926-019-0844-7
- Rudwaleit M, Listing J, Brandt J, et al. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis. 2004;63(6):665-70. doi:10.1136/ard.2003.016386
- Dougados M, Lucas J, Desfleurs E, et al. Impact of disease activity outcome measures reporting in the medical records of patients with axial spondyloarthritis on the retention rates of biological treatment: the example of secukinumab use in daily practice in France. RMD Open. 2022;8(1):e002106. doi:10.1136/rmdopen-2021-002106
- Spoorenberg A, van Tubergen A, Landewé R, et al. Measuring disease activity in ankylosing spondylitis: patient and physician have different perspectives. Rheumatology. 2005;44(6):789-95. doi:10.1093/rheumatology/keh595
- Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016;68(2):282-98. doi:10.1002/art.39298
- Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2022;0:1–16. doi: 10.1136/ard-2022-223296
- Machado P, Landewé R, Lie E, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70(1):47. doi:10.1136/ard.2010.138594
- Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77(1):3-17. doi:10.1136/annrheumdis-2017-211734
- Kiltz U, Landewé RBM, van der Heijde D, et al. Development of ASAS quality standards to improve the quality of health and care services for patients with axial spondyloarthritis. Annals of the Rheumatic Diseases 2020;79:193-201. doi: 10.1136/annrheumdis-2019-216034