

Explore the clinical implications of high RF in RA
RA=rheumatoid arthritis;
RF=rheumatoid factor.
RF is an autoantibody that targets the Fc region of IgG antibodies found in many patients with RA. It is a commonly used biomarker of the disease.1 High RF in RA is associated with greater disease activity and increased risk of radiographic progression.2,3 Recent studies have shown that drug clearance and clinical response to some TNFis may be affected by high RF.4,5 Therefore, RF serum levels should be considered when making clinical decisions in patients with RA.2
RF is a risk factor and commonly used biomarker in RA
RA is a chronic inflammatory joint disease leading to cartilage and bone damage, which is associated with high levels of disability, increased morbidity and mortality, and can significantly impact patient quality of life.1,6,7
RF is a physiologic autoantibody found in the serum and synovial fluid of up to 80% of patients with RA and is a commonly used biomarker of this disease.1,8 RF can be used as a diagnostic tool, predictor of drug response, and prognostic indicator.1,9
- In one study, individuals in the general population with high RF* (>100 IU/mL) vs low RF (<25 IU/mL) had10:
-
Up toand
26-fold
greater long-term
risk of RAUp to
32%
10-year absolute
risk of RA
*Ranges for RF positivity and definitions for high RF vary by laboratory or study.
RF plays a key role in RA pathobiology
RF is linked to the cytokine-dependent inflammation underlying RA,8 via its interaction with the Fc region of IgG antibodies and formation of immune complexes.5 RF is found in multiple immunoglobulin isotypes (IgM, IgG, and IgA); however, the most prevalent in RA is IgM-RF. Each IgM-RF molecule can bind to up to 10 IgG1-Fc regions, which aids in immune complex clearance.1,5
ACPA is another autoantibody that presents in most patients with RA.1 ACPA aids in RF binding and immune complex formation, leading to downstream activation of complement.1 These immune complexes contribute to RA when they form at the sites of synovial inflammation.1
RF binding leads to immune complex formation1
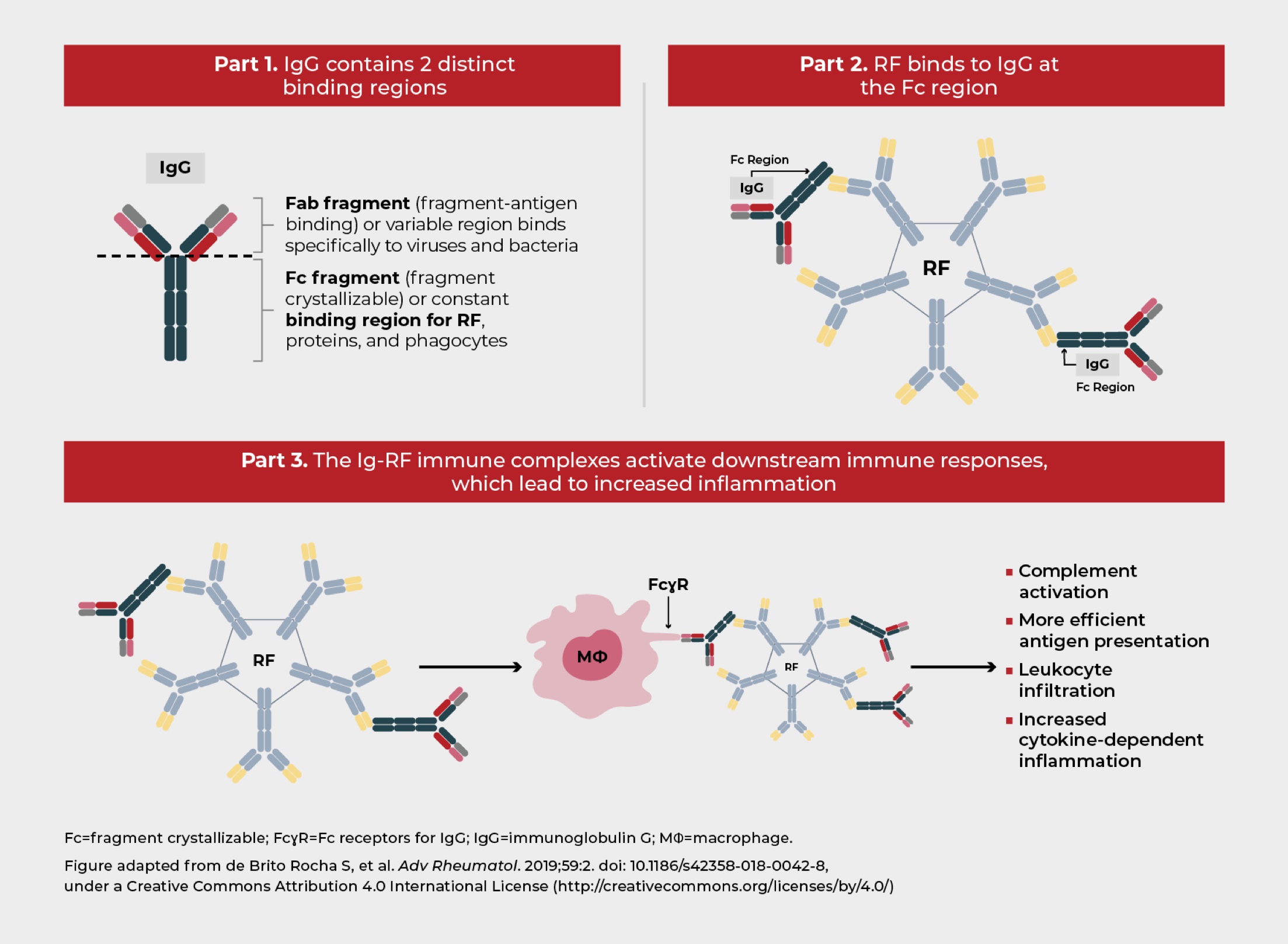
RF and ACPA have been linked with cytokine-dependent inflammation in RA11,12
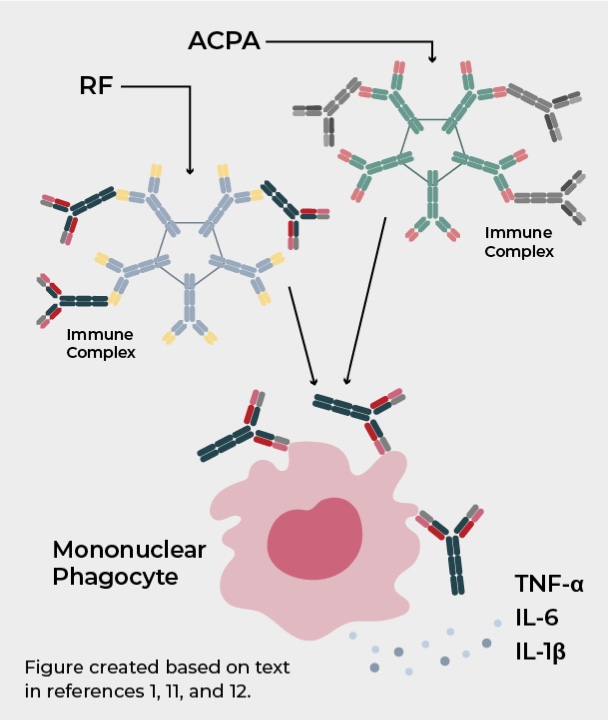
The role of RF in the pathobiology of RA has not yet been fully elucidated; however, it has been proposed that RF potentiates the pathological effects of ACPA. After antigen recognition by these autoantibodies, the immune complex formed can activate mononuclear phagocytes in circulation and joints, promoting the secretion of inflammatory mediators, such as TNF-α, IL-6, and IL-1β. These cytokines have been associated with driving chronic inflammation in RA.11,12
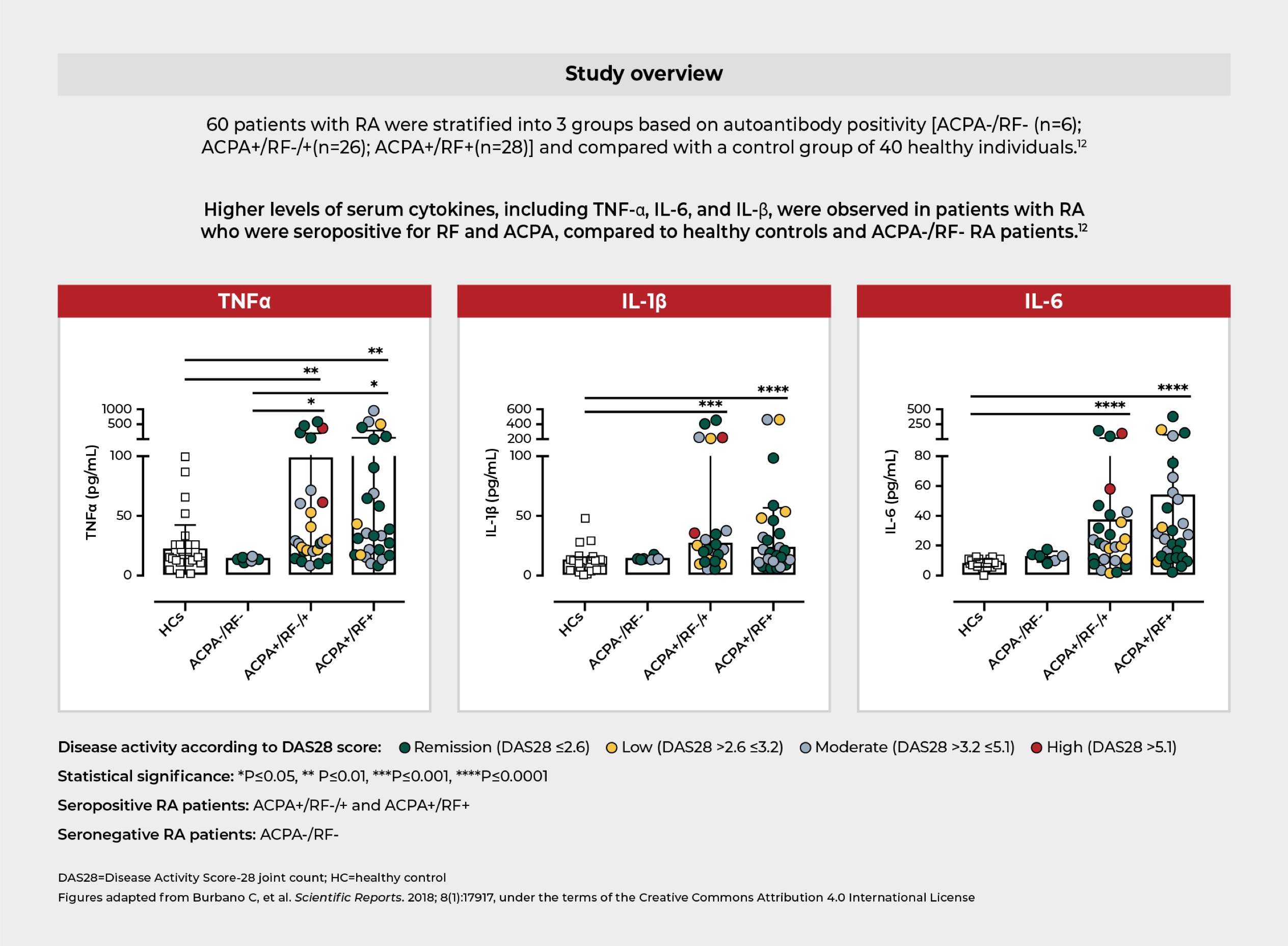
One study showed that increased levels of proinflammatory cytokines associated with RA were observed in patients with high RF and high ACPA levels.12
Systemic inflammatory response in RF and ACPA positive patients is characterized by higher serum levels of RA-associated cytokines12
High RF may lead to more severe disease in patients with RA
High RF is a predictor of poor disease prognosis and is associated with a more aggressive and destructive disease course.5,8
- In patients with RA, having high RF is associated with:
-


Clinical unmet needs in patients with RA and high RF
TNFis are an established first-line biological therapy for RA, with 2 decades of clinical experience. However, many patients with RA have suboptimal clinical responses after initial treatment with TNFis.16,17

Longitudinal cohort study of over 2,000 patients with RA showed that up to 40% of patients treated with TNFis over a 2-year period may respond inadequately because of primary non-response, loss of response, or intolerance.16,17
- High RF in RA has been identified as a factor associated with poorer clinical outcomes after treatment with some TNFis4
-
 Reduced rates of
Reduced rates of
remission Lower likelihood of disease
Lower likelihood of disease
improvement Poorer response to certain
Poorer response to certain
TNFi treatments
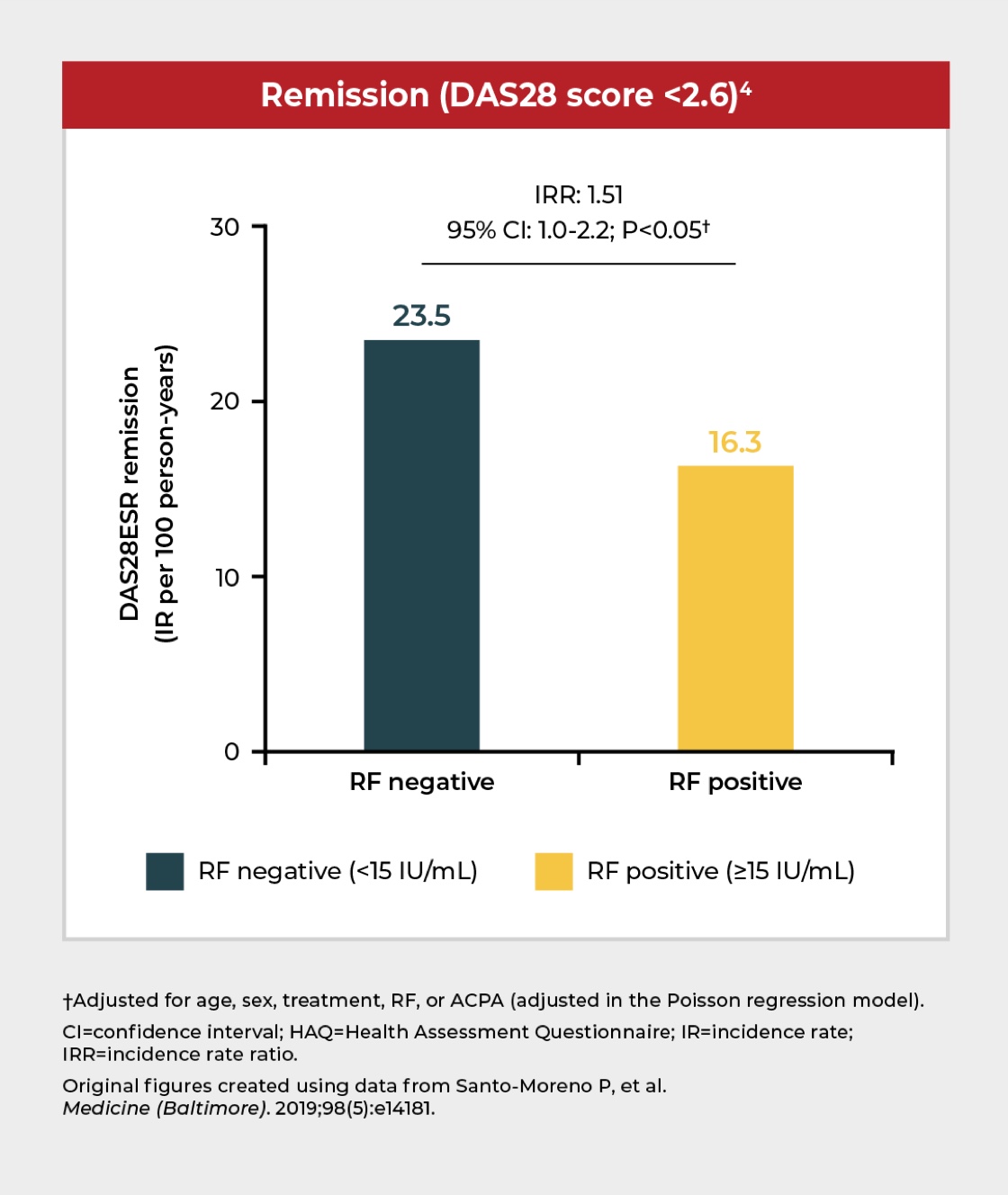
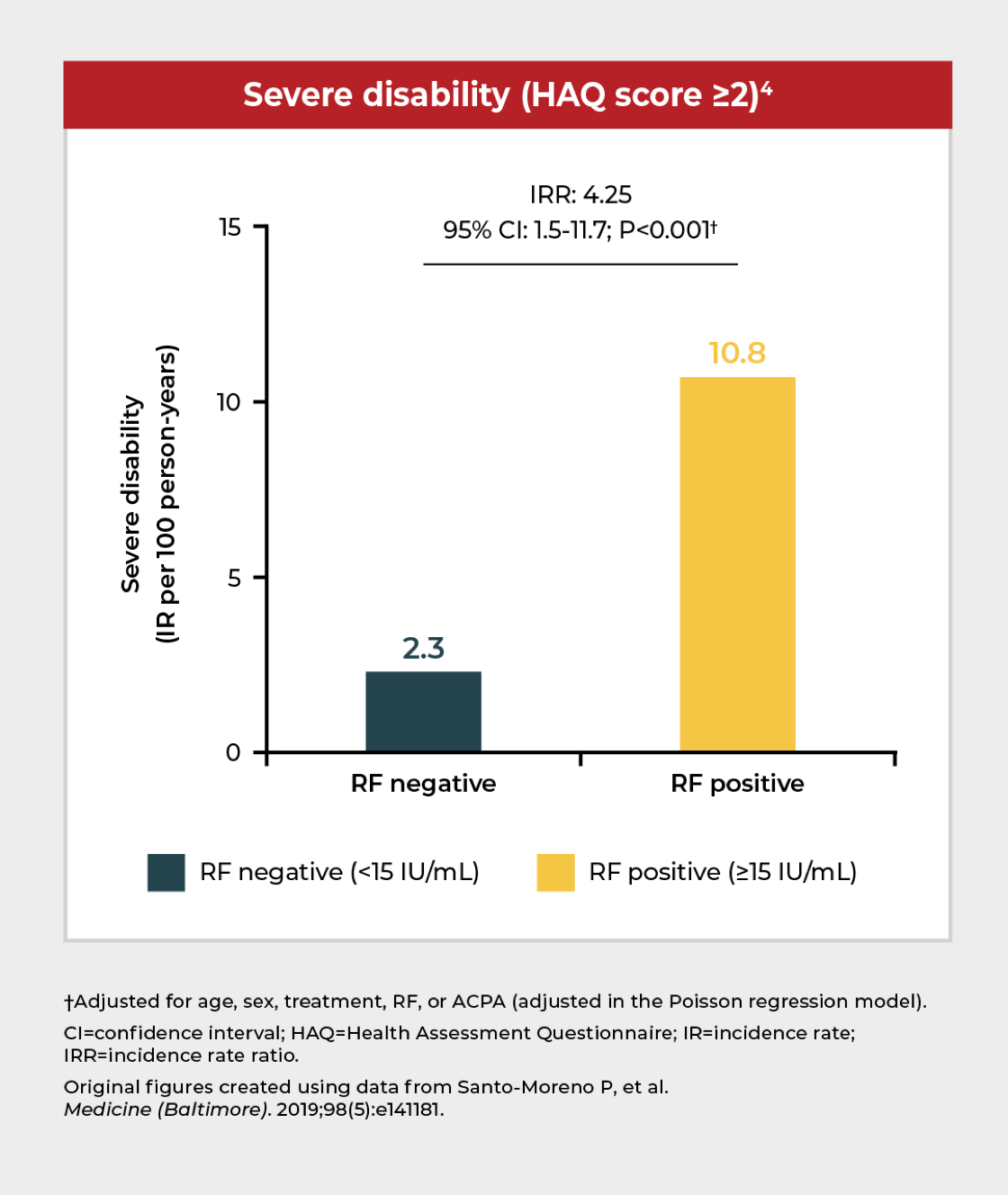
RF positivity is associated with severe disability and lower likelihood of disease remission
In a cohort study of ~400 patients receiving TNFi treatment,* RF positivity (≥15 IU/mL) was associated with severe disability and a lower likelihood of disease remission compared with RF-negative patients.4
*Including etanercept, infliximab, adalimumab, or golimumab.
Additional studies have similarly demonstrated that poor clinical responses to some TNFis may be linked to high RF levels:
In a UK cohort study of patients with RA (N=642), baseline serum IgM-RF positivity (≥40 U/μL) was associated with reduced response to TNFi treatment.18
Data from a Japanese registry (n=1,151) showed that RF positivity was predictive of TNFi discontinuation due to ineffectiveness and was inversely associated with DAS28ESR remission.19


The importance of considering RF levels when making clinical decisions
Several studies have shown that poor clinical outcomes are associated with high RF levels in patients with RA treated with some TNFis.4,18,19 These findings suggest that RF serum levels should be measured and used to help inform treatment strategies and clinical decision making.2
Further, there is evidence that suggests that response to treatment could be related to differences in TNFis.20 Learn more about these differences and discover the latest data/insights on clinical outcomes in RA patients with high RF.
Get more insights on the clinical implications of high RF in RA

Prof. Joseph Smolen, chairman of the Division of Rheumatology at the Medical University of Vienna
“We are striving to find biomarkers that allow us to understand which patients will respond to particular therapies. And I believe that rheumatoid factors may be such a biomarker.”
Watch a video of Professor Joseph Smolen at the Virtual Showcase, Rheum Now Live, 2024.
Become a member of the RheuMuseum and be the first to know about new exhibits
Sign up to stay in the loop on new exhibits about clinical research, important updates, and other educational opportunities.
- de Brito Rocha S, Baldo DC, Andrade LEC. Clinical and pathophysiologic relevance of autoantibodies in rheumatoid arthritis. Adv Rheumatol. 2019;59:2. doi: 10.1186/s42358-018-0042-8
- Aletaha D, Alasti F, Smolen JS. Rheumatoid factor, not antibodies against citrullinated proteins, is associated with baseline disease activity in rheumatoid arthritis clinical trials. Arthritis Res Ther. 2015;17(1):229. doi: 10.1186/s13075-015-0736-9
- Aletaha D, Alasti F, Smolen JS. Rheumatoid factor determines structural progression of rheumatoid arthritis dependent and independent of disease activity. Ann Rheum Dis. 2013;72(6):875-880. doi: 10.1136/annrheumdis-2012-201517
- Santos-Moreno P, Sánchez G, Castro C. Rheumatoid factor as predictor of response to treatment with anti-TNF alpha drugs in patients with rheumatoid arthritis: Results of a cohort study. Med (Baltimore). 2019;98:5. doi: 10.1097/MD.0000000000014181
- Tanaka Y, Takeuchi T, Haaland D, et al. Efficacy of certolizumab pegol across baseline rheumatoid factor subgroups in patients with rheumatoid arthritis: Post-hoc analysis of clinical trials. Int J Rheum Dis. 2023;26(7):1248-1259. doi: 10.1111/1756-185X.14699
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023-2038. doi: 10.1016/S0140-6736(16)30173-8
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094-1108. doi: 10.1016/S0140-6736(10)60826-4
- Mathsson L, Lampa J, Mullazehi M, et al. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arth Res Ther. 2006;8(3):1-10. doi: 10.1186/ar1926
- Bobbio-Pallavicini F, Caporali R, Alpini C, Avalle S, et al. High IgA rheumatoid factor levels are associated with poor clinical response to tumour necrosis factor alpha inhibitors in rheumatoid arthritis. Ann Rheum Dis. 2007;66:302-307. doi: 10.1136/ard.2006.060608
- Nielsen SF, Bojesen SE, Schnohr P, et al. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: a prospective cohort study. BMJ. 2012; Sep 6;345:e5244. doi: 10.1136/bmj.e5244
- Mueller AL, Payandeh Z, Mohammadkhani N, et al. Recent advances in understanding the pathogenesis of rheumatoid arthritis: new treatment strategies. Cells. 2021; Nov 4;10(11):3017. doi: 10.3390/cells10113017
- Burbano C, Rojas M, Muñoz-Vahos C, et al. Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci Rep. 2018;8(1):17917. doi: 10.1038/s41598-018-36335-x
- Cojocaru M, Cojocaru IM, Silosi I, et al. Extra-articular manifestations in rheumatoid arthritis. Maedica (Bucur). 2010;5(4):286-291
- Sobhy N, Ghoniem SA, Eissa BM, et al. Disease characteristics in high versus low titers of rheumatoid factor or anti-citrullinated peptide antibody in rheumatoid arthritis patients. Egypt Rheumatol. 2022;44(4):325-328. doi: 10.1016/j.ejr.2022.04.004
- ElSherbiny D. Frequency and predictors of extra-articular manifestations in patients with rheumatoid arthritis. EJHM. 2019;76(5):4062-4067. doi: 10.21608/ejhm.2019.42300
- Taylor PC, Matucci Cerinic M, Alten R, et al. Managing inadequate response to initial anti-TNF therapy in rheumatoid arthritis: optimising treatment outcomes. Ther Adv Musculoskelet Dis. 2022;14:1-14. doi: 10.1177/1759720X221114101
- Aaltonen KJ, Ylikylä S, Tuulikki Joensuu J, et al. Efficacy and effectiveness of tumour necrosis factor inhibitors in the treatment of rheumatoid arthritis in randomized controlled trials and routine clinical practice. Rheumatology. 2017;56:725-735. doi: 10.1093/rheumatology/kew467
- Potter C, Hyrich KL, Tracey A, et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann Rheum Dis. 2009;68(1):69-74. doi: 10.1136/ard.2007.084715
- Ogawa Y, Takahashi N, Kaneko A, et al. Association between seropositivity and discontinuation of tumor necrosis factor inhibitors due to ineffectiveness in rheumatoid arthritis. Clin Rheumatol. 2019;38:2757-2763. doi: 10.1007/s10067-019-04626-x
- Nakayama Y, Watanabe R, Murakami K, et al. Differential efficacy of TNF inhibitors with or without the immunoglobulin fragment crystallizable (Fc) portion in rheumatoid arthritis: the ANSWER cohort study. Rheumatol Int. 2022;42(7):1227-1234. doi: 10.1007/s00296-021-05086-w