
Delve into the significance of axSpA outcome measures
Measuring disease activity: Composite vs individual domain measures
Given the number of different clinical manifestations and heterogeneity of disease presentation of axSpA, several different measures of disease activity have been developed, including composite and domain-specific assessments.1,2
Routine use of disease activity measures in clinical practice needs to be implemented to help more patients achieve personalized disease outcome goals.1,3
ASAS Quality Standards recommend the following as a priority area: Disease activity of patients with axSpA is monitored under the supervision of a rheumatologist with validated composite scores at least every 6 months.4
Composite disease outcome measures
Composite measures allow for the estimation of overall disease activity and disease burden across several clinical manifestations and often include factors important to both physicians and patients1,5:
- Severity of clinical manifestations (such as joint swelling and tenderness, peripheral arthritis, psoriasis)
- Patient’s assessments of back pain and fatigue
- Physical functioning assessments, including morning stiffness
- Health-related quality of life scores
Many different composite outcome measures are available, and their use in clinical practice often depends on physician and patient preference, the patient’s disease manifestations, time available, and ease of use.1,2,5
Routine use of composite measures, including ASDAS, ASAS, and BASDAI, are recommended by the ASAS-EULAR guidelines.1,*
BASDAI=Bath Ankylosing Spondylitis Disease Activity Index.
*Examples of commonly used disease measures and not an exhaustive list of all available measures.


LECTURE SERIES WITH DR. ALEXIS OGDIE
Delve into 3 common composite outcome measures for axSpA
In this video, Dr. Alexis Ogdie gives her expert insights into composite outcome measures that are useful in both research and a clinical setting, and how measuring many aspects of axSpA together can help guide treatment decisions.
Ankylosing Spondylitis Disease Activity Score (ASDAS)
ASDAS is an ASAS-EULAR guideline-recommended composite index that assesses disease activity in axSpA.1 It includes five activity variables: total back pain (BASDAI Q2), duration of morning stiffness (BASDAI Q6), PtGA NRS, peripheral pain/swelling (BASDAI Q3), and an objective laboratory measure of inflammation, either CRP [ASDAS-CRP] or ESR [ASDAS-ESR].1,5
Considerations
- Designed for use in clinical practice and recognized by ASAS-EULAR guidelines as a preferred measure for axSpA5
- Digital aids are available online to help with score calculations6
- Considers both patients’ and physicians’ perception of the level of disease activity1,5
ASDAS-CRP=ASDAS-C-reactive protein; ASDAS-ESR=ASDAS-erythrocyte sedimentation rate; NRS=numerical rating scale; PtGA=patient global assessment.
Assessment of SpondyloArthritis international Society (ASAS)
ASAS response is an assessment that measures short-term change in AS through patient-reported outcomes.1,5,9 It includes four independent domains: PtGA in the past week, back pain in the past week (VAS or NRS), physical function (BASFI), and inflammation (BASDAI Q5 and Q6).5,7
Considerations
- Although it includes important aspects of feasibility and discrimination, it does not include objective measures of inflammation, such as MRI and CRP5,7
AS=ankylosing spondylitis; BASDAI=Bath Ankylosing Spondylitis Disease Activity Index; BASFI=Bath Ankylosing Spondylitis Functional Index; CRP=C-reactive protein; MRI=magnetic resonance imaging; NRS=numerical rating scale; PtGA=patient global assessment; VAS=visual analog scale.
Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)
BASDAI is an established patient-reported outcome measure that defines disease activity in AS and includes six questions: fatigue (BASDAI Q1), spinal pain (BASDAI Q2), joint pain/swelling (BASDAI Q3), areas of localized tenderness (enthesitis BASDAI Q4), morning stiffness severity (BASDAI Q5), and morning stiffness duration (BASDAI Q6).5
Considerations
- Easy and quick to administer and commonly used in clinical practice5
- BASDAI scores do not correlate well with symptoms and clinical measurements of disease activity and/or MRI scores1,5
AS=ankylosing spondylitis.
Additional disease outcome measures in axSpA
axSpA is a heterogeneous disease and, as a result, a large array of disease outcomes measures have been developed and validated to define and monitor it.1,2 These include physician- and patient-administered measures, composite and individual measures of disease activity according to disease manifestation and quality of life, and ability to carry out activities of daily living.2,5 In addition to those measures summarized above, other measures include (but are not limited to) those listed. View additional measures.
Domain-specific disease outcome measures
Many measures specific to individual manifestations of axSpA have been developed, and their use in clinical practice depends on several factors, such as individual patient needs, time available, and ease of use.2,5,12-14
Individual domain measures can provide an assessment of the impact of a significant symptom, such as pain, which can have an important impact on a patient’s life.15
Commonly used domain-specific measures include BASFI, MASES, and nocturnal back pain NRS.15,*
MASES=Maastricht Ankylosing Spondylitis Enthesitis Score; NRS=numerical rating scale.
*Examples of commonly used disease measures and not an exhaustive list of all available measures.


LECTURE SERIES WITH DR. ALEXIS OGDIE
Examine 3 common domain-specific outcome measures for axSpA
Join Dr. Alexis Ogdie as she explains the strengths and weaknesses of 3 different outcome measures designed to assess a specific aspect of axSpA disease, and how they can be used in clinical practice.
Bath Ankylosing Spondylitis Functional Index (BASFI)
BASFI is a patient-administered questionnaire that evaluates functional limitation on a VAS or NRS.11 It consists of eight questions relating to functional anatomy (bending, reaching, changing position, standing, turning, and climbing steps) and 2 questions to assess the patient’s ability to cope with everyday life.11
Considerations
- Scoring is simple and quick (~3 minutes), with questions that are easy to understand2,11
- BASFI is a frequently used functional index for assessment of AS patients2
- Although it was designed for patients with AS in clinical trials, it can be used in clinical practice2
AS=ankylosing spondylitis; NRS=numerical response scale; VAS=visual analog scale.
Maastricht Ankylosing Spondylitis Enthesitis Score (MASES)
MASES is a measurement that assesses the presence or absence of enthesitis at 13 sites (entheseal indices) in patients with AS.12
Considerations
- Feasible to use in clinical practice13
- Reflects disease activity as assessed by other measures in SpA, but does not correlate well with changes in quality of life measures16
- Can be administered without the physician applying pressure at all enthesis sites, which may reduce the burden to patients13
Nocturnal back pain NRS
Nocturnal back pain NRS is a patient-reported outcome assessment of nocturnal back pain in patients with AS.13 Questions cover levels of pain in the spine and duration of recall (current, past 24 hours, past week, etc.).13
Considerations
- The tool is inexpensive and broadly accessible13
AS=ankylosing spondylitis.
Additional disease outcome measures in axSpA
axSpA is a heterogeneous disease and, as a result, a large array of disease outcome measures have been developed and validated to define and monitor it.1,2 These include physician- and patient-administered measures, composite and individual measures of disease activity according to disease manifestation and quality of life, and ability to carry out activities of daily living.2,5 In addition to those measures summarized above, other measures include (but are not limited to) those listed. View additional measures.
Where do we go from here?
Routine implementation of more stringent measures of axSpA disease activity is needed to clearly define disease manifestations and to help raise the bar in helping more patients achieve optimal disease outcome goals.5,8
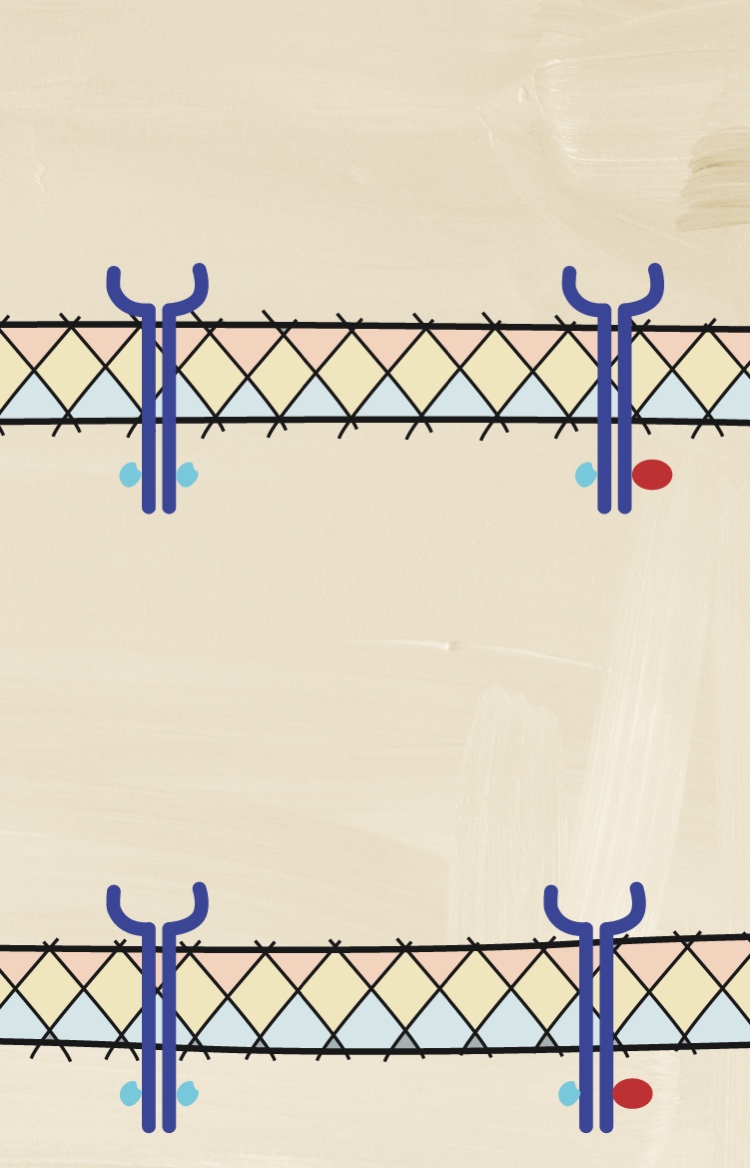
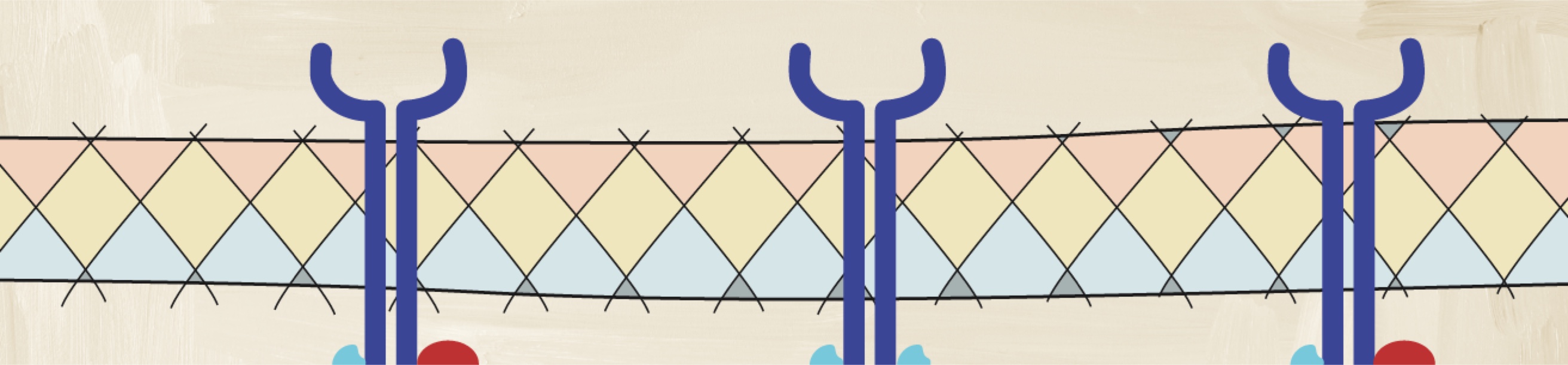
How may a deeper understanding of cytokines help with assessing disease activity?
Understanding the pathologic processes leading to axSpA is important to understanding its heterogeneous nature.


NEXT: TAKE THE PSA TOUR
Pathobiology overview and the role of cytokines in PsA
You have finished the axSpA tour. Be sure to visit the PsA tour and discover how several different cytokines have distinct roles in driving inflammation in PsA.
PREVIOUS ROOM
Setting higher goals
Become a member of the RheuMuseum and be the first to know about new exhibits
- Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2022;0:1–16. doi: 10.1136/ard-2022-223296
- Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S). Arthritis Care Res. 2011;63 Suppl 11:S47-58. doi:10.1002/acr.20575
- Ward MM, Deodhar A, Akl EA, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2016;68(2):282-98. doi:10.1002/art.39298
- Kiltz U, Landewé RBM, van der Heijde D, et al. Development of ASAS quality standards to improve the quality of health and care services for patients with axial spondyloarthritis. Annals of the Rheumatic Diseases 2020;79:193-201. doi: 10.1136/annrheumdis-2019-216034
- Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77(1):3-17. doi:10.1136/annrheumdis-2017-211734
- Assessment of SpondyloArthritis International Society (ASAS). ASDAS calculator. https://www.asas-group.org/instruments/asdas-calculator/. Accessed September 2022
- Machado P, Landewé R, Lie E, et al. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70(1):47. doi:10.1136/ard.2010.138594
- van der Heijde D, Joshi A, Pangan AL, et al. ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis. Rheumatology. 2016;55(1):80-8. doi:10.1093/rheumatology/kev267
- Anderson JJ, Baron G, van der Heijde D, et al. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 2001;44(8):1876-86. doi:10.1002/1529-0131(200108)44:8<1876::aid-art326>3.0.co;2-f
- Brandt J, Listing J, Sieper J, et al. Development and preselection of criteria for short term improvement after anti-TNF alpha treatment in ankylosing spondylitis. Ann Rheum Dis. 2004;63(11):1438-44. doi:10.1136/ard.2003.01671
- Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21(12):2281-5.
- Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis. 2003;62(2):127-32. doi:10.1136/ard.62.2.127
- Ogdie A, Duarte-García A, Hwang M, et al. Measuring outcomes in axial spondyloarthritis. Arthritis Care Res. 2020;72 Suppl 10:47-71. doi:10.1002/acr.24266
- Kiltz U, Braun J. Assessments of functioning in patients with axial spondyloarthritis. J Rheum Dis. 2020;27(1):22-29. doi:10.4078/jrd.2020.27.1.22
- Lukas C, Dougados M, Combe B. Factors associated with a bad functional prognosis in early inflammatory back pain: results from the DESIR cohort. RMD Open. 2016;2(1):e000204. doi:10.1136/rmdopen-2015-000204
- Hartung W, Nigg A, Strunk J, et al. Clinical assessment and ultrasonography in the follow-up of enthesitis in patients with spondyloarthritis: a multicenter ultrasound study in daily clinical practice. Open Access Rheumatol. 2018;10:161-169. doi:10.2147/oarrr.s179472